Treating Genetically Defined Diseases
Cogent’s lead drug candidate, bezuclastinib, is designed to target exon 17 mutations found within the KIT receptor tyrosine kinase, including KIT D816V. When KIT D816V remains in a perpetual ‘on’ state causing mast cells, a type of white blood cell, to accumulate in various internal organs including the bone marrow. The result is an orphan disease called Systemic Mastocytosis (SM).
Exon 17 mutations have also been found in advanced Gastrointestinal Stromal Tumors (GIST), which have a strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a powerful new treatment option for patients with both of these diseases.
Our Approach
SYSTEMIC MASTOCYTOSIS
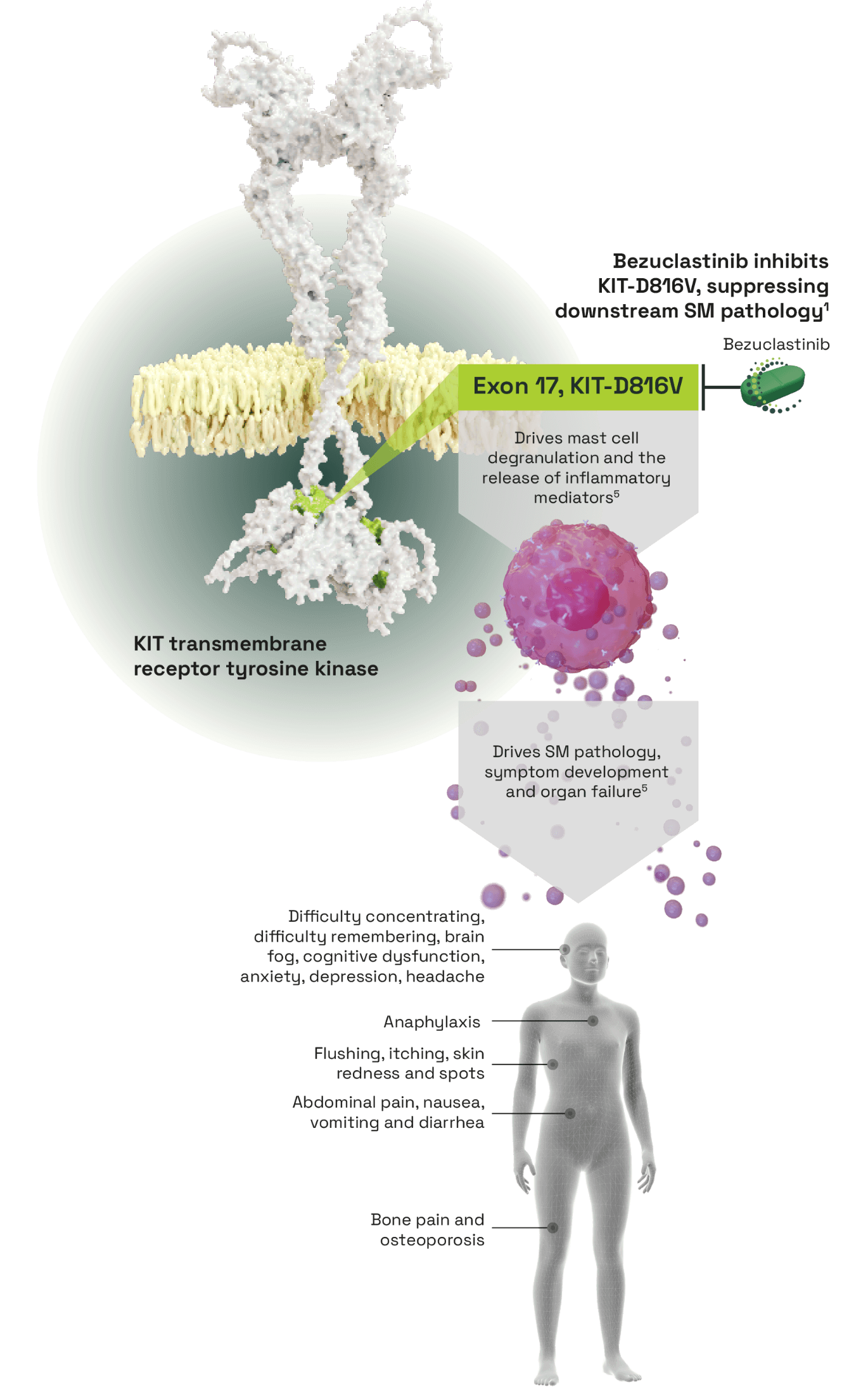
Bezuclastinib, a tyrosine kinase inhibitor (TKI) selectively targeting KIT activation loop mutations in systemic mastocytosis (SM)1
Disease Background
- The KIT receptor tyrosine kinase is found on the surface of certain cell types2
- KIT is activated by binding to its ligand, stem cell factor, which triggers multiple essential signaling cascades2
- KIT-D816V mutations cause independent constitutive activation of the KIT receptor and dysregulation and proliferation of mast cells2
- Mast cells accumulate abnormally around the body and release inflammatory mediators causing widespread pathological effects3
- SM is categorized as advanced or non-advanced based on the symptom burden and level of organ involvement3
The unmet need in SM
- TKIs targeting activation loop mutations like KIT-D816V are promising treatments for SM but are limited in optimal dosing due to off-target toxicities and associated clinical liabilities3
Bezuclastinib, a novel type I TKI3,4
- Selectively and potently inhibits KIT-D816V, an activation loop mutation
- No activity against other closely related kinases and reduced off-target toxicities
References
- DeAngelo D et al. EHA2022 Hybrid Congress. Poster P1049. Vienna, 2022.
- Piris-Villaespesa M and Alvarez-Twose I. Front Pharmacol 2020; 11(443):doi: 10.3389/fphar.2020.00443. eCollection 2020.
- Goncalves I et al. ASH Annual Meeting & Exposition. Poster 4569.1. San Diego, 2024.
- Guarnieri A et al. AACR Annual Meeting. Poster 147. New Orleans, 2022.
- Gilreath JA et al. Clin Pharmacol 2019; 11: 77-92.
GIST
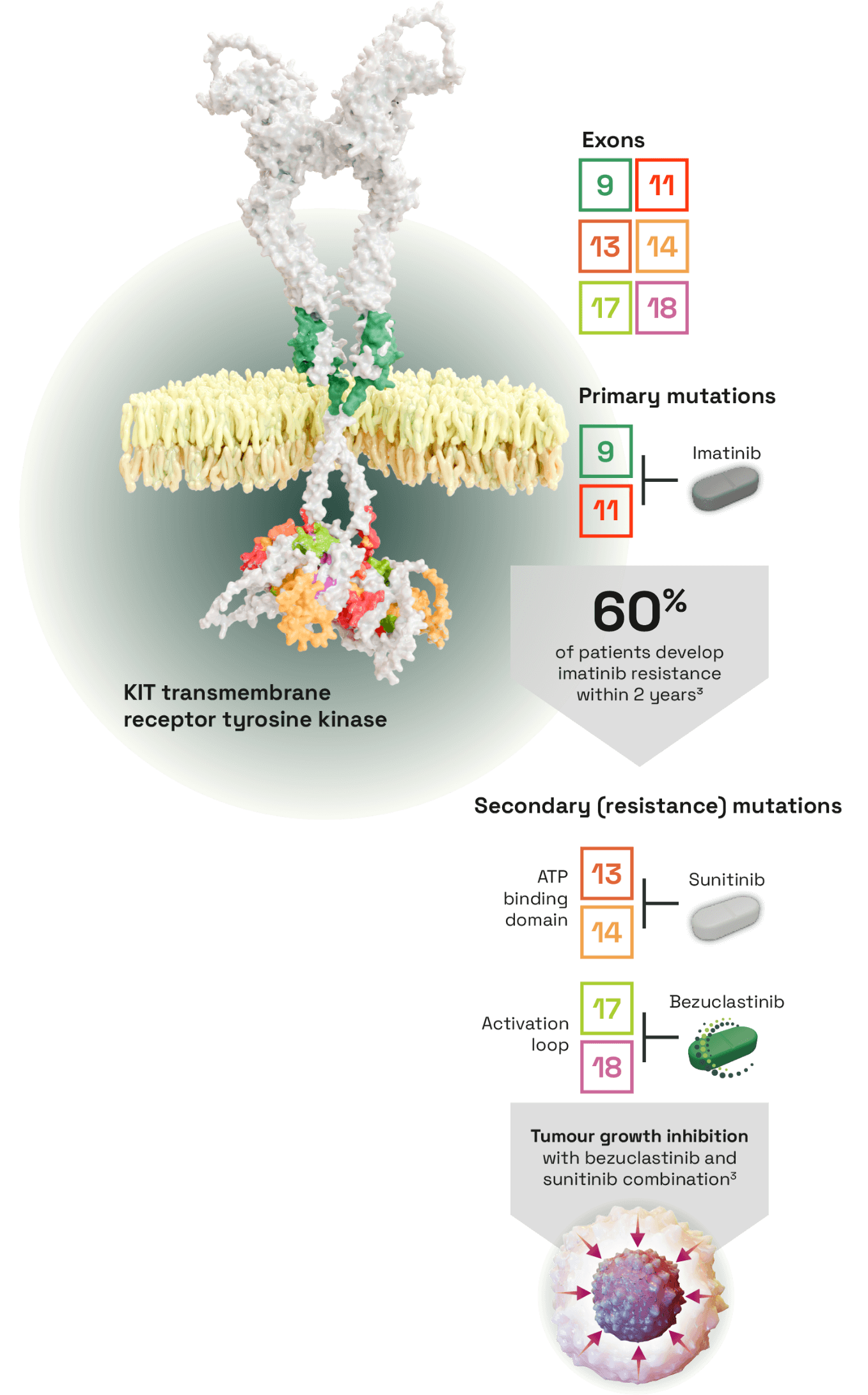
Bezuclastinib, a tyrosine kinase inhibitor (TKI) selectively targeting KIT activation loop mutations in GIST1
Disease Background
- The KIT receptor tyrosine kinase is found on the surface of certain cell types, and is activated by binding to its ligand, stem cell factor, which triggers multiple essential signaling events2
- KIT gene mutations drive interstitial cells of Cajal proliferation and GIST pathology due to ligand-independent, constitutive activation of the KIT receptor2
- Primary mutations in exons 9 or 11 are found in >80% of GISTs which can be targeted with first-line imatinib3
- However, 60% of patients develop imatinib resistance within 2 years due to secondary mutations, commonly in exons 13, 14, 17 or 183
The unmet need in GIST
- No single therapy targets all common primary and secondary mutations. Different TKIs target different KIT mutations but toxicities prevent them from being used in combination3
Bezuclastinib, a novel type I TKI1,3
- Selectively inhibits KIT with limited activity on closely related kinases
- Fewer off-target effects and toxicities expected (edema, hypertension, and pleural effusion)
- Allows investigation in combination with sunitinib, covering common primary and secondary KIT mutations
References
- Guarnieri A et al. AACR Annual Meeting. Poster 147. New Orleans, 2022.
- Nemunaitis J et al. Future Oncol 2020; 16(1): 4251–64.
- Wagner A et al. ASCO Annual Meeting. Poster 459. Chicago, 2024.

